
The electrochemical CO2 reduction reaction (eCO2RR) to produce value-added multi-carbon (C2+) products, especially ethanol, represents an effective strategy for sustainable energy conversion and achieving carbon neutrality. Although copper (Cu) is the standard electrocatalyst for C2+ product formation, it has inherent limitations related to its density, mechanical strength, susceptibility to corrosive environments, and the ongoing challenge of attaining high C2+ selectivity and operational stability. This situation calls for the exploration of innovative catalytic architectures.
To tackle these significant challenges, a recent study led by Prof. Abudukeremu Kadier and Prof. MA Pengcheng from the Xinjiang Technical Institute of Physics and Chemistry (XTIPC) of the Chinese Academy of Sciences, introduces basalt fiber fabric (BFF) as a novel and high-performance support for Cu electrocatalysts. This study was published in the journal Energy.
BFF was selected for its unique properties: low density, exceptional mechanical strength, outstanding chemical inertness, and inherent corrosion resistance, making it a promising alternative to traditional catalyst supports. Additionally, Xinjiang province has abundant natural reserves of basalt, positioning BFF as a potentially low-cost material.
By leveraging these intrinsic properties, the researchers engineered a robust catalytic platform through a simple electroless Cu deposition process. This process resulted in a uniformly deposited conductive material with a substantial Cu loading of 96.79 wt.%. The Cu-deposited BFF showed a significantly lower density (3.08±0.4 g/cm3) compared to bulk Cu (8.96 g/cm3), while also demonstrating markedly improved mechanical properties (breaking forces of 3308±25 N in the warp direction and 665±20 N in the weft direction) and high electrical conductivity (4.81 × 105 S/m before eCO2RR, slightly decreasing to 4.58 × 105 S/m after the reaction).
Comprehensive electrochemical characterization in CO2-saturated potassium bicarbonate (KHCO3) electrolytes (ranging from 0.1 M to 2.0 M) revealed that the developed electrocatalyst achieved a current density of 25.93 mA/cm² with an exceptional Faradaic efficiency (FE) of 97.01% for ethanol production at an applied voltage of -0.8 V versus the reversible hydrogen electrode (RHE) in a conventional H-type cell.
Mechanistic analysis indicated that the catalyst primarily favored a complex twelve-electron pathway for C2H5OH synthesis, though minor competing reactions were also noted: a two-electron process for CO formation (0.42% FE) and an eight-electron process for CH4 formation (0.43% FE), along with a limited two-electron hydrogen evolution reaction (H2, 2.14% FE). The significantly higher FE for ethanol highlights the optimized surface properties and reaction conditions that promote the multi-electron CO2 reduction pathway.
Notably, increasing the electrolyte concentration to 1.5M KHCO3 substantially improved catalytic performance, achieving an impressive current density of 184.51 mA/cm² and a remarkable FE of 98.02% for ethanol, demonstrating unprecedented selectivity.
Moreover, the Cu-deposited BFF exhibited outstanding operational longevity, retaining 98.8% and 99.6% of its initial current density after 100 hours of continuous electrolysis in 0.1M and 1.5M KHCO3 electrolytes, respectively. This exceptional stability over 100 hours of continuous operation can be attributed to the inherent mechanical strength and corrosion resistance of the BFF support, which effectively maintains the structural integrity and catalytic activity of Cu.
This research establishes BFF as a novel, multifunctional, and scalable support material for the rational design of robust and high-performance Cu-based electrocatalysts for efficient CO2 conversion, representing a significant advancement toward sustainable chemistry and climate change mitigation.

Preparation and structural characterization of Cu-deposited BFF catalyst. (Image by XTIPC)
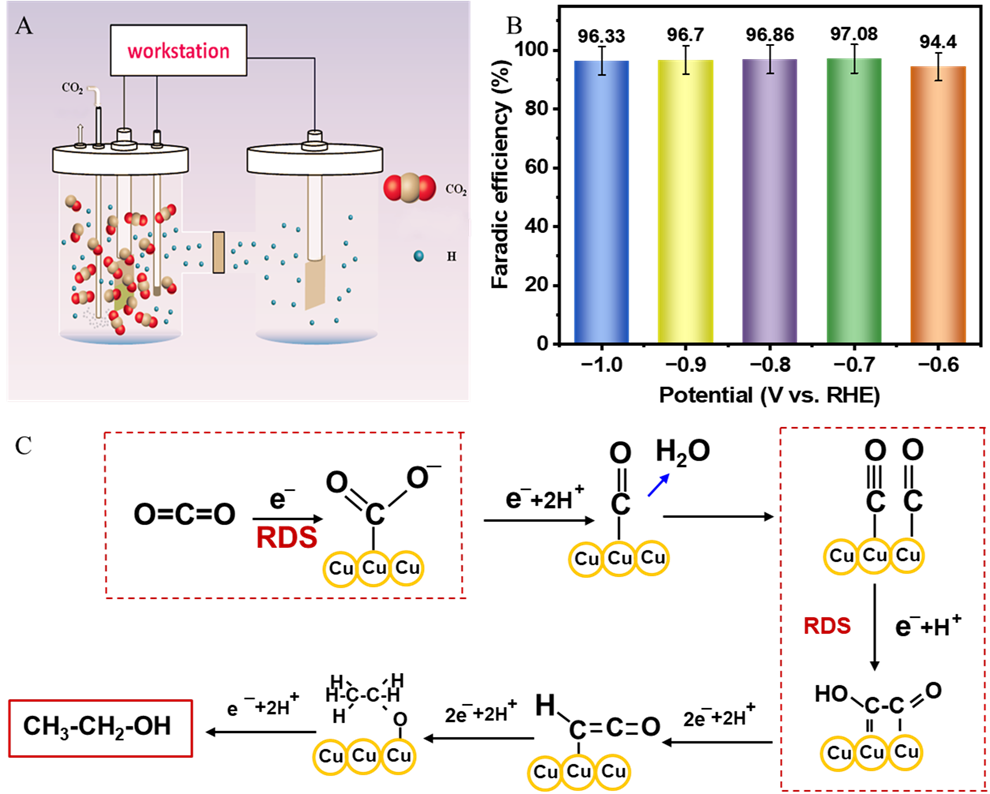
Evaluation of eCO2RR on Cu-deposited BFF catalyst. (Image by XTIPC)

86-10-68597521 (day)
86-10-68597289 (night)

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

